Download Brochure
If you want to learn more about the training, check out the official training brochure!

What is included?
- You have 12 months time as of reception of the learning material to learn, do the exam and get your certification
- Certification and examination fees are included in the price of the training course
-
Training material containing over 450 pages of information and practical examples will be distributed
- An Attestation of Course Completion worth 31 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course.
- If candidates fail the exam, they can retake it for free within 12 months of the course completion.
- + 20% reduction on the first year subscription for our all-in-one ISMS/GRC management solution
Why should you take this training course?
During this training course, you will acquire the knowledge and skills to plan and carry out internal and external audits in compliance with ISO 19011 and ISO/IEC 17021-1 certification process.
Based on practical exercises, you will be able to master audit techniques and become competent to manage an audit program, audit team, communication with customers, and conflict resolution.
After acquiring the necessary expertise to perform this audit, you can sit for the exam and apply for a “PECB Certified ISO 13485 Lead Auditor” credential. By holding a PECB Lead Auditor Certificate, you will demonstrate that you have the capabilities and competencies to audit organizations based on best practices.
Who should attend?
The ISO 13485 Lead Auditor training course is intended for:
- Auditors seeking to perform and lead Medical Devices Quality Management System (MDQMS) certification audits
- Managers or consultants seeking to master a Medical Devices Quality Management System audit process
- Individuals responsible for maintaining conformance with Medical Devices Quality Management System requirements
- Technical experts seeking to prepare for a Medical Devices Quality Management System audit
- Expert advisors in Medical Devices Quality Management
Training course structure
Module 1: Introduction to Medical Devices Quality Management Systems (MDQMS) and ISO 13485
- Course objectives and structure
- Standards and regulatory frameworks
- Certification process
- Fundamental principles of Medical Devices Quality Management Systems
- Medical Devices Quality Management System (QMS)
Module 2: Audit principles, preparation and launching of an audit
- Fundamental audit concepts and principles
- Audit approach based on evidence and risk
- Initiating the audit
- Stage 1 audit
- Preparing the stage 2 audit (on-site audit)
- Stage 2 audit (Part 1)
Module 3: On-site audit activities
- Stage 2 audit (Part 2)
- Communication during the audit
- Audit procedures
- Creating audit test plans
- Drafting audit findings and non-conformity reports
Module 4: Closing the audit
- Documentation of the audit and its review
- Closing the audit
- Evaluating action plans by the auditor
- Beyond the initial audit
- Managing an internal audit programme
- Competence and evaluation of auditors
- Closing the training
Certification Exam
Learning objectives
Upon successfully completing the training course, you will be able to:
- Understand the operations of a Medical Devices Quality Management System based on ISO 13485
- Acknowledge the correlation between ISO 13485 and other standards and regulatory frameworks
- Understand an auditor’s role to: plan, lead and follow-up on a management system audit in accordance with ISO 19011
- Learn how to lead an audit and audit team
- Learn how to interpret the requirements of ISO 13485 in the context of a MDQMS audit
- Acquire the competencies of an auditor to: plan an audit, lead an audit, draft reports, and follow-up on an audit in compliance with ISO 19011
Examination
The “PECB Certified ISO 13485 Lead Auditor” exam fully meets the requirements of the PECB Examination and Certification Programme (ECP). The exam covers the following competency domains:
- Domain 1: Fundamental principles and concepts of a Medical Devices Quality Management System (MDQMS)
- Domain 2: Medical Devices Quality Management System (MDQMS)
- Domain 3: Fundamental audit concepts and principles
- Domain 4: Preparation of an ISO 13485 audit
- Domain 5: Conducting an ISO 13485 audit
- Domain 6: Closing an ISO 13485 audit
- Domain 7: Managing an ISO 13485 audit program
Duration: 3 hours
Location: Online through the PECB app OR in person in one of the PECB exam centers
Preparation: PECB Exam Preparation Guides
Language: The exam is available in multiple other languages and does not need to be taken in the same language as the training material. Additional time can be requested when your native language is not available in your mother tongue (to be requested by candidates on the exam day)
Retake: In case you fail the exam, you can retake it within 12 months following the initial attempt for free
For specific information about the exam type, languages available, and other details, please visit the List of PECB Exams and the Examination Rules and Policies.
Certification
After successfully completing the exam, you can apply for the credentials shown on the table below. You will receive a certificate once you comply with all the requirements related to the selected credential.
For more information about the ISO 13485 certifications and the PECB certification process, please refer to the Certification Rules and Policies.
The requirements for PECB Auditor Certifications are:
| Credential | Exam | Professional experience | MS audit/assessment experience | Other requirements |
| PECB Certified ISO 13485 Provisional Auditor | PECB Certified ISO 13483 Lead Auditor Exam or equivalent | None | None | Signing the PECB Code of Ethics |
| PECB Certified ISO 13485 Auditor | PECB Certified ISO 13483 Lead Auditor Exam or equivalent | Two years: One year of work experience in Medical Devices Quality Management | Audit activities: a total of 200 hours | Signing the PECB Code of Ethics |
| PECB Certified ISO 13485 Lead Auditor | PECB Certified ISO 13483 Lead Auditor Exam or equivalent | Five years: Two years of work experience in Medical Devices Quality Management | Audit activities: a total of 300 hours | Signing the PECB Code of Ethics |
| PECB Certified ISO 13485 Senior Lead Auditor | PECB Certified ISO 13483 Lead Auditor Exam or equivalent | Ten years: Seven years of work experience in Medical Devices Quality Management | Audit activities: a total of 1,000 hours | Signing the PECB Code of Ethics |
Note: PECB Certified Individuals who do possess the Lead Implementer and Lead Auditor Credentials are qualified for the respective PECB Master Credential, given they have taken 4 additional Foundation Exams which are related to this scheme. For more detailed information about the Foundation Exams and the overall Master Requirements, please visit the following link: https://pecb.com/en/master-credentials
To be considered valid, these audits should follow best audit practices and include the following activities:
- Audit planning
- Audit interview
- Managing an audit program
- Drafting audit reports
- Drafting non-conformity reports
- Drafting audit working documents
- Documentation review
- On-site audit
- Follow-up on non-conformities
- Leading an audit team
Contact us on [email protected] if you have other questions
Start for free now!
Streamline your GRC work using our all-in-one management solution and get access to our network of local specialists

Start for free now!
Streamline your GRC work using our all-in-one management solution and get access to our network of local specialists
Ask any question about our products
Check our PECB frequently asked question (FAQ) page or contact us with the form below:
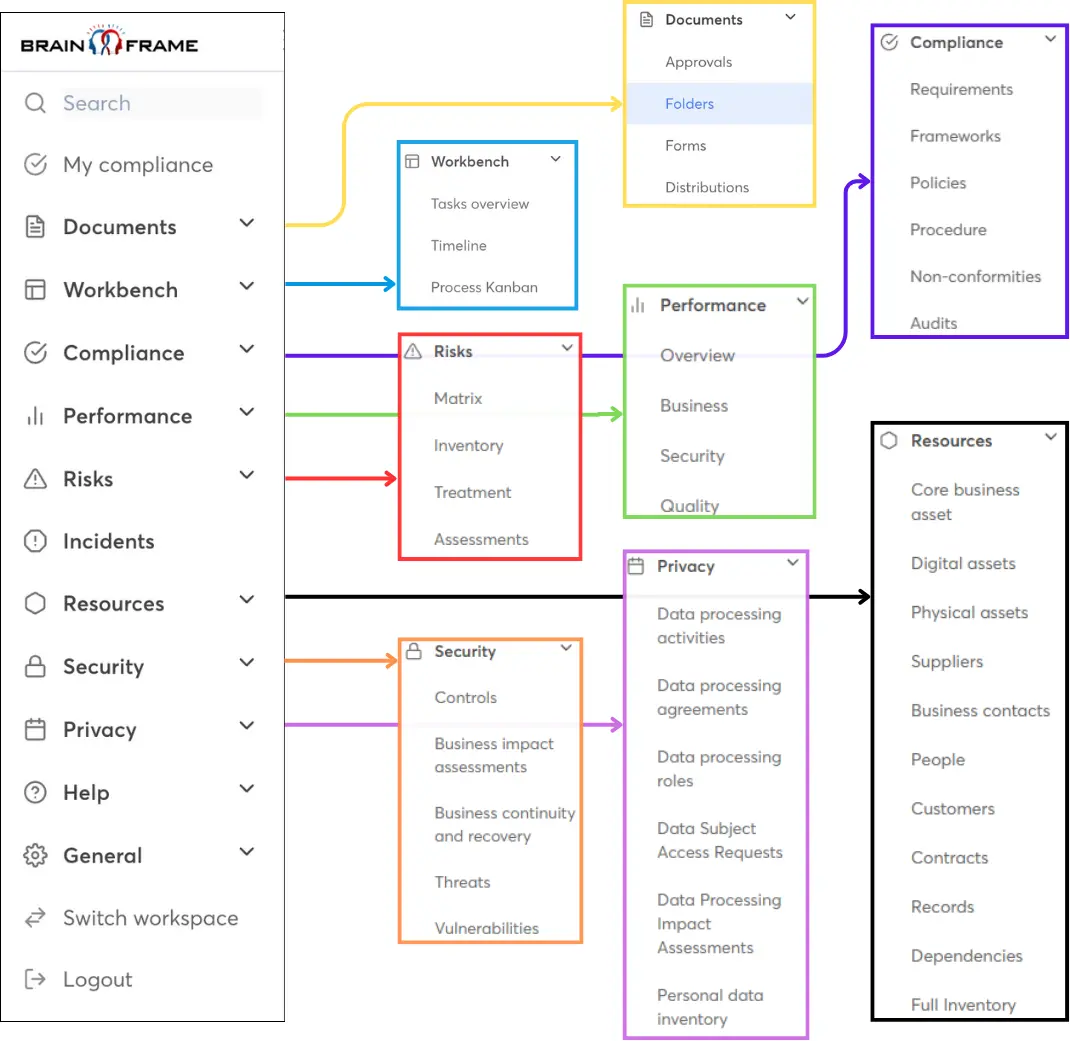
Streamline your GRC work using our all-in-one management solution and get access to our network of local specialists
Start your free account
